Ag + Hcl Net Ionic Equation
Write the equation and balance it. OH HOCl H 2 O OCl B.
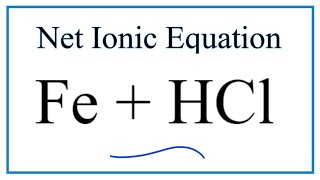
How To Write The Net Ionic Equation For Fe Hcl Fecl2 H2 Iron Hydrochloric Acid Youtube
Na HOCl NaCl OH D.
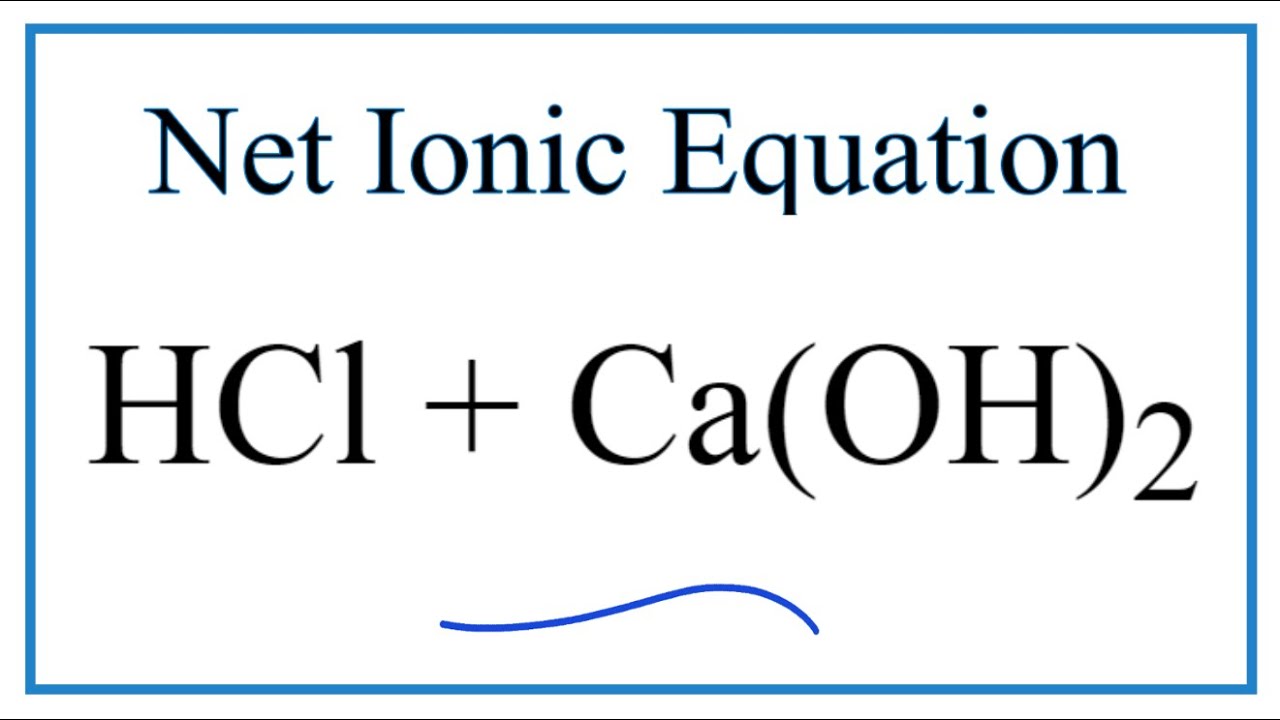
. First we balance the molec. Aqueous potassium carbonate reacts with aqueous silver nitrate to precipitate silver carbonate. There are three main steps for writing the net ionic equation for AgNO3 HCl HNO3 AgCl Silver nitrate Hydrochloric acid.
The question-writer is lying to you. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. OH 2 OCl HOCl O C.
The net ionic equation cancels out spectator ions that have the same formula and state of matter on either side of the equation Select the NET ionic equation for the reaction between HCl and NaOH. Write the balanced net ionic equation for HClaq reacting with H 2 SO 4 aq The problem is that these two compounds both acids do not react. SNO DRUGS TITRANT INDICATOR ELECTRODE REFERENCE ELECTRODE 1 Amoxicillin sodium Mercuric nitrate PtHg Hg-mercurous sulphate 2 Acebutalol HCl NaOH Glass SCEAg-AgCl 3 Dextromorphan HCl NaOH Glass SCEAg-AgCl 4 DiphenoxylateHCl Ethanolic NaOH Glass SCEAg-AgCl 5 Disulfiram Ag2NO3 Silver SCE 6 Hydralazine HCl Potassium iodate.
Sulfuric acid reacts to neutralize aqueous sodium. 2Nas 2HClaq - 2NaClaq H 2 g Step 2. Aqueous sodium hydroxide reacts with aqueous copperII sulfate to precipitate copperII hydroxide.
Ag in solution 2NH 3 in solution AgNH 3 2 in solution In this step you are just generating the first equilibrium above the solubility equilibrium for AgCl. Use uppercase for the first character in the element and lowercase for the second character. IronIi Sulfide Hydrogen Chloride Ferrous Chloride Hydrogen Sulfide.
NaOH HOCl H 2 O NaCl 8. Which is the net ionic equation for the reaction that occurs when NaOH is added to this buffer. Write a balanced ionic equation Ag aq Cl-aq AgCls Example.
The balanced equation will appear above. When you added HCl a concentrated Cl-ion solution to AgNO 3 a white precipitate of AgCl formed. FeS 2HCl FeCl2 H2S might be an ionic equation.
Fe Au Co Br C O N F. Ag aq Cl-aq AgCl s Do you understand this. However notice how the question is phrased to imply that the two compounds do in fact react.
Write the ionic equation for the word equation. An aqueous solution of barium nitrate BaNO 3 2aq is mixed with dilute sulfuric acid H 2 SO 4aq. Take the test now.
H HOCl H 2 OCl E. Ag aq Cl-aq AgCl s If HCl aq is the source of Cl-aq and AgNO 3aq is the source of Ag aq then the net ionic equation is. Ionic Equation Worksheet Write balanced molecular total ionic and net ionic equations for each of the following.
Sodiums hydrochloric acidaq - sodium chlorideaq hydrogeng Solution. 1 mole of solid IronIi Sulfide reacts with 2 moles of aqueous Hydrogen Chloride to form 1 mole of aqueous Ferrous Chloride and 1 mole of solid Hydrogen Sulfide. Only compounds that are aqueous are split into ions 2Nas.
Assuming equal concentrations of conjugate base and acid which one of the following mixtures is suitable for making a buffer solution with an. Ionic charges are not yet supported and will be ignored. A Haq OHaq H2Ol.
One more example not NR then some final comments. Write the complete molecular complete ionic. Double Displacement Metathesis Net Ionic Equation.

How To Write The Net Ionic Equation For Hcl Sr Oh 2 H2o Srcl2 Youtube

How To Write The Net Ionic Equation For Ag Hc Youtube
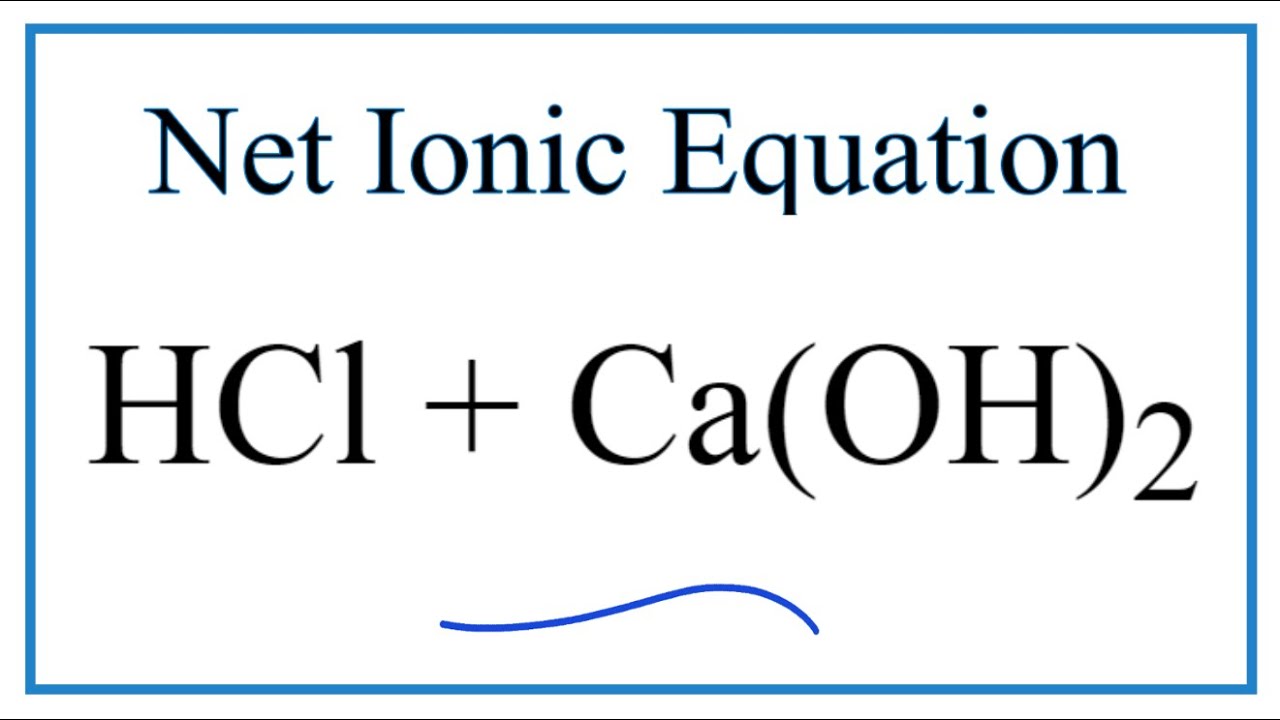
How To Write The Net Ionic Equation For Hcl Ca Oh 2 Cacl2 H2o Youtube
Comments
Post a Comment